
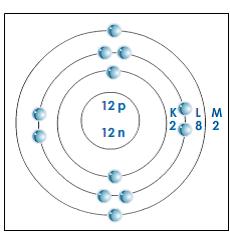

Like the other alkaline earth metals (group 2 of the periodic table) it occurs naturally only in combination with other elements and it almost always has an oxidation state of +2. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. The Periodic Table shows Magnesium has an atomic mass of 24.31. Magnesium is a chemical element with the symbol Mg and atomic number 12. Atomic number tells us the number of protons in the atom, and as we discussed before the number of electrons will equal the number of protons in a neutral atom - so now we know both of these numbers! The mass number is the sum of the number of protons AND neutrons in the atom, as we know the number of protons (from the atomic number), all we have to do now is subtract that from the mass number to find the number of neutrons. Atomic number 12 Atomic mass 24.305 atomic Master unit Symbol Mg Number of subatomic particles 12 protons, 12 neutrons, and 12 electrons position on the. Presentation on theme: QQ: A magnesium atom has 12 protons and 15 neutrons. There are two electrons in the outermost orbit of the magnesium atom. To draw the structure of a specific atom (here we're doing Magnesium), we need two numbers from the periodic table the atomic number and the mass number (the bigger of the two numbers). Neutrons and neutral anyway (the name is a hint!) so they don't have an effect on charge - their only purpose is to physically separate the positive charges of protons in the nucleus, so they don't all repel each other. Protons have a positive charge and electrons have a negative charge, so for an atom to have a neutral charge there needs to be an equal number of these two. Its cosmic abundance is estimated as 9. Atoms are made up of protons and neutrons in the nucleus, and electrons surrounding the nucleus in shells. Magnesium is the eighth most abundant element in Earth ’s crust (about 2.5 percent) and is, after aluminum and iron, the third most plentiful structural metal.


 0 kommentar(er)
0 kommentar(er)
